Depression is a really tough illness to deal with. Even though there have been a ton of treatments developed over the years, none have proven to be the ultimate solution. Common medications like SSRIs and SNRIs were thought to be improvements over older treatments.
Yet, they still come with drawbacks like sexual issues, stomach problems, weight gain, issues when trying to stop the medication, and problems with sleep. These side effects often discourage people from sticking with the treatment and can even prevent them from getting fully better.
Imagine trying to climb a mountain, but never quite making it to the top. That’s what it’s like for many people dealing with major depressive disorder (MDD) when their treatment doesn’t fully work. This is a problem because any leftover symptoms could mean the depression might come back.

So, scientists are always on the lookout for new, more effective treatments. Some have tried creating medications that target specific parts of the brain involved with depression, but these haven’t always worked out as hoped.
Interestingly, one promising approach is to focus on our body’s internal clock, or “circadian rhythms”. Do you know how you feel off when you’re jet-lagged or pull an all-nighter? That’s because your circadian rhythms are out of whack. And guess what, people dealing with depression often have disturbed circadian rhythms too.
This has led to the creation of a new type of antidepressant called Valdoxan. Unlike other treatments, Valdoxan works in a unique way: it acts like melatonin, a hormone that regulates your sleep-wake cycle, and also blocks a specific type of serotonin receptor in the brain. By doing this, it aims to restore balance to your internal clock and improve your mood.
This is pretty revolutionary in the world of depression treatments. Preliminary studies show promising results, but there’s more to learn about how it can help people achieve a better quality of life. In a way, Valdoxan represents a new hope in our ongoing struggle to conquer depression.
Good quality remission is rarely achieved with conventional antidepressants, suggesting that targets other than the monoaminergic system hold the key to effective drug therapy. A novel example is circadian rhythm regulation, based on the finding that circadian rhythms are disturbed in depressed patients and that such disturbance is an important factor in depressive episode outcome.
Valdoxan (agomelatine) is the first melatonergic antidepressant. As the first antidepressant to be an agonist at melatonergic MT1 and MT2 receptors and an antagonist at serotonergic 5HT2C receptors, it has a unique receptor profile for regulating disturbed circadian rhythms.
It has demonstrated antidepressant efficacy against placebo in the short and long term, irrespective of disease severity, and has also outperformed venlafaxine and sertraline on several rating scales, especially those reflecting clinical practice.
Efficacy is associated with unique clinical benefits such as non-sedative regulation of the sleep-wake cycle and improved daytime functioning as early as the first treatment week.
Valdoxan does not depress libido or sexual function, nor induce antidepressant discontinuation syndrome or weight gain, and it is well tolerated. These features make it a truly novel approach to depression, providing early symptomatic relief within a framework of complete and sustained remission.
Medicographia. 2009;31:175-181. (see French abstract on page 181)
Keywords: internal clock; circadian rhythm; unique receptor profile; antidepressant efficacy; remission; discontinuation syndrome; sexual function; body weight; Valdoxan (agomelatine)
Pharmacologic properties of Valdoxan: a truly innovative antidepressant therapy
Receptor Profile

Valdoxan binds strongly to cloned human MT1 and MT2 receptors with affinity (Ki) values of 0.10 ±0.1 nM and 0.12 ±0.02 nM.7 Its estimated Ki for 5HT2C receptors is 710 nM (pKi=6.15 ±0.04).8 Inhibitory concentration 50 (IC50) values for MT1, MT2, and 5HT2C receptors are 0.13, 0.47, and 270 nM (Figure 1). Screening of over 80 receptors and enzymes has revealed no other relevant affinities.
Thus unlike the SSRI fluoxetine, Valdoxan (10-50 mg/kg intraperitoneal [IP]) affects neither 5HT1A receptor density nor basal electrophysiologic characteristics and responses to 5HT1A receptor stimulation in the dorsal raphe (presynaptic receptors) or hippocampus (postsynaptic receptors).9
Nor does Valdoxan (10-40 mg/kg IP) affect serotonin outflow under acute and chronic conditions.8,10 In freely moving rats it increases dopamine outflow in the frontal cortex and norepinephrine levels in the frontal cortex and the hippocampus.
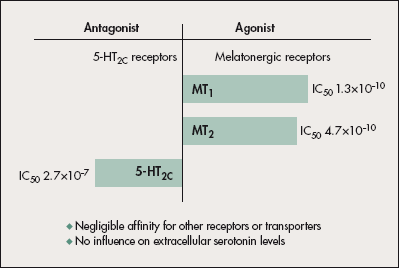
Figure 1. Receptor profile of Valdoxan. Valdoxan is an agonist at melatonergic
MT1/MT2 receptors and an antagonist at 5HT2C receptors. Its affinity for other receptors or transporter sites is negligible. IC50, inhibitory concentration–50.
This effect appears to be mediated by 5HT2C receptor antagonism. No other increase in dopamine has been observed in other brain areas, specifically the nucleus accumbens or striatum.
Valdoxan Demonstrates Efficacy in All Animal Models of Depression
Valdoxan (10-50 mg/kg per os [PO] or IP) has shown antidepressant efficacy in a comprehensive and validated battery of animal models11-14: despair test, olfactory bulbectomy, transgenic mice with glucocorticoid receptor deficiency, unavoidable aversive light stimulus, learned helplessness, and chronic mild stress.
In the latter model, a melatonergic agonist abolishes the antidepressant effect observed in the evening but not that observed in the morning, clearly showing that the antidepressant efficacy of Valdoxan requires both melatonergic and 5HT2C receptors.
Resynchronization of Circadian Rhythms

Resynchronization in Models of Circadian Rhythm Disturbance
In rats, single or repeated dosing with Valdoxan (5- 10 mg/kg PO) resynchronizes locomotor activity in models of jet lag, blindness, and delayed-phase sleep; it also resynchronizes circadian rhythms in two therapeutic models: aging and trypanosome infection.15-18
Resynchronization of Circadian Rhythms in An Animal Model of Depression
In a naturalistic and well-validated animal model of depression (the subdominant tree shrew), Valdoxan (40 mg/kg PO) resynchronizes body temperature, while restoring body weight and urinary cortisol. 19 Melatonin and 5HT2C antagonists have no effect in the same model.20
Resynchronization of Circadian Rhythms in Healthy Volunteers
In healthy volunteers, chronic exposure to a therapeutic dose of Valdoxan (50 mg) induced an approximate 2-hour phase advance of body temperature and cortisol rhythm and an increase in plasma growth hormone. No effect on polysomnography variables is observed.21 These results are reinforced by the impact of Valdoxan in depressed patients (see next section: Clinical studies).
Other Activities
Single dosing with Valdoxan (10-40 mg/kg IP) has proven anxiolytic activity in the elevated plus maze, social defeat model, Geller-Seifert and Vogel conflict tests, and social interaction tests. 5HT2C antagonists are effective in these models and melatonin is effective only in the elevated plus maze test.10,22 Chronic evening dosing with Valdoxan (40 mg/kg IP) increases cell proliferation, neurogenesis, and newly-formed neuron survival in the hippocampus.23
Mechanism of Action: A Novel Approach to Depression
Valdoxan has proven efficacy in animal models of depression in which melatonin and 5HT2C receptor antagonists have no effect. The preclinical studies show that this efficacy is dependent on both melatonergic receptor agonism and 5HT2C receptor antagonism.
The results suggest that neither the affinity for either receptor alone or a simple combination of the two pharmacologic activities suffice to provide antidepressant activity. The receptors could therefore be acting in synergy (rather than in combination) to achieve antidepressant efficacy via complete regulation of circadian rhythms.
Several lines of evidence substantiate this proposed mode of action: (i) high MT1, MT2, and 5HT2C receptor density in the suprachiasmatic nucleus and hippocampus24-26; (ii) involvement of all these receptors in the regulation of circadian rhythm27,28; (iii) circadian expression of MT1 and 5HT2C receptors29,30; and (iv) beneficial effects on sleep by melatonergic agonists and slow-wave sleep promotion by 5HT2C receptor antagonists.27,31 This mechanism of action represents a true innovation in antidepressant therapy.
- Clinical studies: Valdoxan is a potent antidepressant, providing faster, fuller, and more sustained relief.
Three short-term pivotal studies have demonstrated antidepressant efficacy versus placebo, while comprehensive evaluations have shown that this efficacy is sustained across all grades of depression. Efficacy has also been evaluated in head-to-head comparisons versus venlafaxine and sertraline.
Superior Efficacy to Placebo: Short-Term Studies

Dose-Ranging Study
This was an international, double-blind, randomized, phase 2, parallel-group efficacy study over 8 weeks in 711 patients with MDD (including bipolar II disorder) of Valdoxan 1-5 mg or 25 mg given in the evening versus placebo using paroxetine 20 mg as the internal validator.32
The efficacy endpoint (mean decrease in total score on the 17-item Hamilton Rating Scale for Depression [HAM-D17]) confirmed the efficacy of Valdoxan 25 mg versus placebo (Ä 2.57; P=0.034), as did the secondary endpoints: Montgomery-Åsberg Depression Rating Scale (MADRS; P=0.016), Clinical Global Impression– Severity (CGI-S) scale (P=0.049), number of responders (61.5% vs 46.3%; P=0.036), and time to first response (P=0.008).
Flexible Dose Studies
Two international, double-blind, randomized, parallel-group studies confirmed the antidepressant efficacy of Valdoxan 25 mg versus placebo over 6 weeks.33,34 The dose could be increased up to 50 mg if improvement was insufficient (based on predetermined cut-offs on the HAM-D17 and CGI scales) after 2 weeks.
The design was unique insomuch as investigators and patients were blinded to the criteria for the dose increase and to the increase itself, which was performed via an interactive voice system.
In both studies (n=23833 and n=21234), Valdoxan was more effective than the placebo in terms of the mean HAM-D score in both the total population (∆=3.44; P<0.001 [Figure 2] and ∆=2.3; P=0.026) and dose-adjusted population (∆=3.71; P=0.018 and ∆=3.13; P=0.045). Secondary endpoints showed improvement with Valdoxan in CGI-S score, number of responders, and time to first response survival analysis.
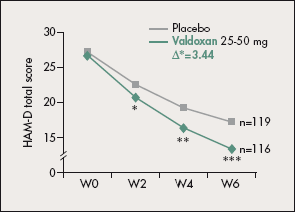
Figure 2. Mean Hamilton Rating Scale for Depression (HAM-D) total scores (adjusted for center and baseline; last observation carried forward) in the Valdoxan (25-50 mg) and placebo groups of a randomized, double-blind, parallel-group trial. *P<0.05; **P<0.01; ***P<0.001.
After reference 33: Olié JP, Kasper S. Efficacy of Valdoxan, an MT1/MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol. 2007;10:661-673.
Copyright © 2007, CINP.
Efficacy in Severe Depression
In all three placebo-controlled pivotal studies, Valdoxan showed significant efficacy in the severe subpopulation, irrespective of the severity criteria used (baseline HAM-D score ≥25 or HAM-D score ≥25 and CGI score ≥5). Meta-analysis of the pooled study data (296 patients randomized to Valdoxan and 295 to placebo) showed that when the population was divided into subgroups using increasing cut-off HAM-D scores (stepwise from HAM-D ≥24 to HAM-D ≥30) at inclusion, antidepressant efficacy persisted irrespective of baseline severity (∆=2.06; P=0.021 and ∆=4.45; P=0.025 for HAM-D cut-offs of 22-25 and >30) (Figure 3).35
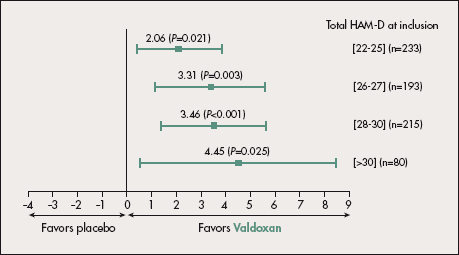
Figure 3. Pooled results of positive placebo-controlled studies on Valdoxan showing antidepressant efficacy in severely depressed patients. Hamilton Rating Scale for Depression (HAM-D) difference from placebo (6-8 weeks; last observation carried forward). The efficacy of Valdoxan 25-50 mg is maintained whatever the degree of severity at inclusion.
After reference 35: Montgomery SA, Kasper S. Severe depression and antidepressants. Focus on a pooled analysis of placebo-controlled studies on agomelatine. Int Clin Psychopharmacol. 2007;22:283-291.
Copyright © 2007, Lippincott Williams and Wilkins.
Superior Efficacy to Placebo: Long-Term Studies
A relapse prevention study randomized responders to Valdoxan 25-50 mg after 8-10 weeks to continue Valdoxan or placebo for 6 months (or an optional 10 months).36,37 The differences in cumulative relapse rates (21.7% vs 46.6% and 23.9% vs 50%) at both 6 and 10 months, respectively, were highly significant: P<0.0001, with nearly 8 in 10 patients relapse-free on Valdoxan, and risk of relapse half that of placebo. The effect was confirmed in the severely depressed subpopulation.
Efficacy versus Venlafaxine and Sertraline: Short-Term and Long-Term Studies
A head-to-head comparative study randomized patients to Valdoxan 25-50 mg (n=163) and venlafaxine 75-150 mg (n=165) in a flexible dose design for 6 weeks extending up to 6 months.38 Improvement on the CGI-improvement (CGI-I) scale was much greater as early as the first week in the Valdoxan patients (∆=0.39; 95% confidence interval [CI], 0.20-0.58; P<0.0001) and remained so at both 6 weeks (∆=0.32; 95% CI, 0.06-0.58; P=0.016) and 6 months (∆=0.32; 95% CI, 0.04-0.6; P=0.025).
Another head-to-head comparative study randomized patients to Valdoxan 25-50 mg (n=150) and sertraline 50-100 mg (n=157) for 6 weeks up to 6 months.39 The response rate was significantly higher with Valdoxan at week 2 (20% vs 10.9%; P=0.027), and at 6 weeks, the scores on the three assessment scales were significantly superior— HAM-D(∆=1.68;P=0.031), CGI-I (∆=0.29;P=0.023), and CGI-S (∆=0.28; P=0.043). At 6 months, the response rate was significantly higher with Valdoxan (76% vs 63.5%; P=0.017).
Anxiolytic Activity
Valdoxan is not only an effective antidepressant, but it also relieves the associated anxiety, as shown using the Hamilton Anxiety Scale (HAMA) in a dose-ranging study versus placebo (∆=3.43; P=0.011), and in the meta-analysis of positive placebo-controlled studies in which Valdoxan patients scored significantly better versus placebo in the psychic and somatic anxiety items (items 10 & 11) of the HAM-D scale, including patients not on concomitant benzodiazepines.40
Valdoxan also outperformed sertraline in terms of HAMA scores at 6 weeks (∆=2.36; 95% CI, 0.45-4.26; P=0.016).39
Better Quality Remission with Valdoxan
Underlying the antidepressant efficacy of Valdoxan is a property not shared by any other available treatment: regulation of the sleep-wake rhythm, which complements and enhances a number of equally unique clinical benefits ensuring better quality remission.
Sedation, daytime somnolence, sexual dysfunction, antidepressant discontinuation syndrome, and weight gain are all remarkable by their absence of Valdoxan therapy, contributing to excellent overall tolerability.
Regulation of The Sleep-Wake Rhythm: Unique to Valdoxan
As expected from its pharmacological profile, Valdoxan restores the disturbed sleep-wake rhythm, which is one of the earliest and most disabling as- aspects of depression. Valdoxan also eliminates daytime drowsiness.
Two studies have evaluated the effect of Valdoxan on sleep-wake patterns using objective measures (polysomnography) and subjective questionnaires. Other information has come from subanalysis of the HAM-D sleep items in the pivotal studies.41
Objective Studies
In the first study,42 depressed patients received Valdoxan 25 mg in the evening for 42 days under open conditions with regular polysomnography. The results showed increased sleep efficiency (P=0.05), decreased intrasleep awakenings (P=0.041), increased slow-wave sleep (stages 3 and 4) both absolutely (P=0.037) and relative to the total sleep time (P=0.022), absence of change in total rapid eye movement sleep, and gradual improvement in the delta sleep ratio. Redistribution of slow-wave sleep through the night normalized sleep architecture.
The head-to-head comparison versus sertraline used actimetry to demonstrate improvement by the end of week 1 on Valdoxan in the objective sleep-wake parameters sleep efficiency, sleep latency, and sleep-wake time.43
Subjective Studies
A 6-week randomized double-blind study compared the effect of Valdoxan 25-50 mg and venlafaxine 75- 150 mg on subjective sleep quality and integrity of behavior using the Leeds Sleep Evaluation Questionnaire (LSEQ) and Visual Analog Scale (VAS).38
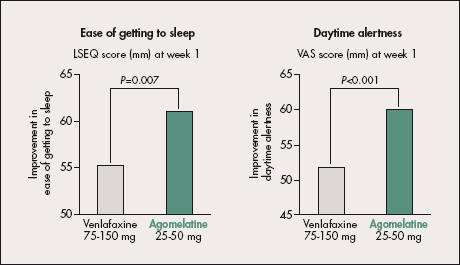
Figure 4. Effects of Valdoxan 25-50 mg and venlafaxine 75-150 mg after 1 week of treatment on the first item in the Leeds Sleep Evaluation Questionnaire (LSEQ; “ease of getting to sleep”; left panel) and on daytime functioning (Visual Analog Scale [VAS]; right panel). Valdoxan regulates sleep better than venlafaxine with improvement in patients’ daytime condition from the first week of treatment. Based on data from reference 38.
It showed significantly greater improvement with Valdoxan in the LSEQ items “getting to sleep” (P=0.007) and “quality of sleep” (P=0.015) in week 1. This effect persisted until the end of treatment.
Significantly greater improvement was also noted in “daytime alertness” and “sensation of well-being” (P_0.001 in week 1), indicating earlier improvement in daytime functioning (Figure 4). The study versus sertraline replicated these findings, showing earlier improvement in “getting to sleep” (P<0.01) and “quality of sleep” (P=0.025) with Valdoxan as early as week 1.
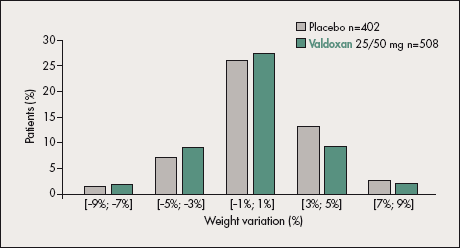
Figure 5. Body weight variation compared between Valdoxan and placebo in the major depressive disorder (MDD) safety sets of long-term placebo-controlled trials. Body weight variation was similarly infrequent in the Valdoxan and placebo groups.
Valdoxan Improves Sleep Through the Night:

Effect on early and middle insomnia and early awakening In the subanalysis of the three pivotal efficacy studies, Valdoxan 25-50 mg was significantly better than placebo on HAM-D sleep subscales in all three phases of sleep: early insomnia (P<0.001), middle insomnia (P=0.015), and early awakening (P=0.006). The difference versus placebo remained significant even without the HAM-D sleep items.41
Preservation of Sexual Functioning
There has been no evidence at any time in its development that Valdoxan impairs sexual function. Sexual emergent adverse events were few and similar on Valdoxan and placebo. A 12-week specific study versus venlafaxine using the Sex Effects Scale in 276 depressive patients, of whom 193 were sexually active at baseline, showed higher scores for pre-orgasm and orgasm with Valdoxan 50 mg than with venlafaxine 150 mg; the same was true in patients who achieved remission (n=111).44
A study in 92 healthy male volunteers randomized to Valdoxan (25 or 50 mg), paroxetine 20 mg, or placebo for 8 weeks confirmed the nil-effect on sexual function using the Psychotropic-Related Sexual Dysfunction-Salamanca Sex Questionnaire (PRSEX-DQ-SALSEX). Sexual dysfunction was markedly less frequent (P<0.0001) in each Valdoxan group (25 mg, 22.7%; 50 mg, 4.8%) than in the paroxetine group (85.7%).
In the placebo group, 8.7% of volunteers reported sexual dysfunction. Volunteer reports of moderate or severe sexual dysfunction were 4.5% for Valdoxan 25 mg, 4.8% for Valdoxan 50 mg, 61.9% for paroxetine 20 mg, and 0% in the placebo group (P<0.0001 for Valdoxan versus paroxetine).45
Absence of The Discontinuation Syndrome
Abrupt discontinuation of conventional antidepressant treatment can trigger some very disturbing symptoms that typically last several days and include nausea, delirium, chills, and sleep disturbance.
This does not occur with Valdoxan. A randomized, double-blind, and placebo-controlled study explored the possibility of discontinuation syndrome with Valdoxan versus paroxetine as the active control. In stable MDD remitters, abrupt cessation of the 12-week treatment with Valdoxan 25 mg induced no symptoms on the Discontinuation Emergent Signs and Symptoms (DESS) checklist after 1week.
Abrupt replacement of paroxetine by placebo, on the other hand, induced many more emergent symptoms than in patients remaining on paroxetine (P<0.001).46
Superior Tolerability to Conventional Antidepressants

Tolerated as well as placebo, including with respect to body weight.
In the short-term studies, the percentages of patients reporting at least one treatment-emergent adverse event (TEAE) on Valdoxan were low and similar to placebo, as were the overall rates of TEAEs and their nature. The most frequent adverse events reported in both groups were headache, nausea, and fatigue.47 No clinically relevant changes in body weight (Figure 5) were observed on Valdoxan 25-50 mg.
Most TEAEs occurred in the first 2 weeks of treatment and were mild to moderate, transient, and self-resolving. Rates of dropout due to adverse events were similar for Valdoxan 25 mg (5.5%), Valdoxan 50 mg (5.2%), and placebo (5.2%).
Valdoxan has a cardiovascular safety profile similar to that of a placebo. Hematology and clinical chemistry show nil of notes. There have been cases of liver enzyme elevation (1.1%; without clinical signs and reversible). Valdoxan has no apparent effect on hormone levels, including prolactin.
Superior Tolerability to Venlafaxine, Paroxetine, and Sertraline
The safety profile of Valdoxan 25-50 mg compared favorably with venlafaxine 75-150 mg over treatment for 6 or 12 weeks.38,44 Fewer patients withdrew due to TEAEs on Valdoxan (4.2% vs 13.2% and 2.2% vs 8.6% after 6 and 12 weeks). There were also fewer total TEAEs, in particular gastrointestinal disturbances and dizziness, on Valdoxan.
An 8-week dose-ranging study 32 showed no difference in tolerability between Valdoxan 25 mg and placebo, whereas patients receiving paroxetine 20 mg reported significantly more gastrointestinal TEAEs than those on placebo (P<0.05) (Table I). Comparison versus sertraline 50-100 mg showed fewer TEAEs causing drop out after 6 weeks with Valdoxan 25-50 mg (2.6% vs 11.3%).
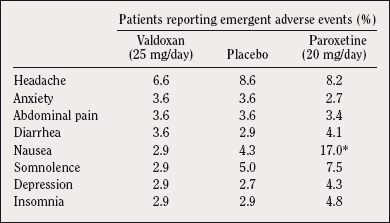
Table I. Main emergent adverse events (% patients in the safety set) reported
by at least 3.5% of patients in any treatment group. Data from all patients who received at least one dose of treatment (even without efficacy evaluation). *P<0.05 (compared with placebo).
Modified from reference 32: Lôo H, Hale A, D’Haenen H. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT2C antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. Int Clin Psychopharmacol.
2002;17:239-247. Copyright © 2002, Lippincott Williams & Wilkins, Inc.
The unique receptor profile accounts for the excellent tolerability of Valdoxan:
- Because Valdoxan has no affinity for serotonin or dopamine transporters, does not increase extracellular serotonin levels, and does not desensitize 5HT1A receptors (unlike TCAs/SSRIs/SNRIs), it does not cause nausea, sexual dysfunction, central nervous system disorders, agitation, nervousness, or insomnia.
- Because Valdoxan has no affinity for muscarinic receptors (unlike TCAs), it does not cause dry mouth, somnolence, blurred vision, urinary retention, or memory deficit.
- Because Valdoxan has no affinity for histamine receptors (unlike TCAs and mirtazapine), it does not cause sedation or weight gain.
- Because Valdoxan has no affinity for noradrenergic receptors or norepinephrine transporters (unlike TCAs, mirtazapine, and SNRIs), it has no cardiovascular effects and does not cause panic attacks or anxiety.
Valdoxan: Superior Treatment Adherence
Proven short-term and long-term efficacy combines these unique clinical benefits to generate better treatment adherence than with the active comparators used in the specific studies. Meta-analysis of the two head-to-head studies showed that more patients continued treatment with Valdoxan beyond 6 months than with venlafaxine and sertraline (69.4% vs 61.5%; P<0.05).
Frequently Asked Questions

Can Valdoxan Be Used in Conjunction with Other Antidepressants?
It’s crucial to consult with your healthcare provider before combining Valdoxan with other antidepressants, as they can provide guidance based on your specific health situation.
How Quickly Can One Expect to See Results with Valdoxan?
The onset of the therapeutic effect of Valdoxan can vary from person to person. It’s important to have regular check-ins with your healthcare provider to monitor progress.
Does Valdoxan Have Any Side Effects?

Like all medications, Valdoxan may have potential side effects. It’s important to discuss these with your healthcare provider before starting treatment.
Can Valdoxan Help Improve Sleep Quality?
Yes, one of the unique benefits of Valdoxan is its ability to help restore the body’s natural sleep-wake cycle, which can improve sleep quality.
Is Valdoxan Suitable for Everyone with Depression?
While Valdoxan has been beneficial for many people with depression, it’s not suitable for everyone. Always consult with a healthcare provider to determine the best treatment plan for you.
What Should I Do if I Miss a Dose of Valdoxan?
If you miss a dose of Valdoxan, it’s important not to double up on your next dose. Instead, take the missed dose as soon as you remember, unless it’s close to the time for your next dose.
How Should Valdoxan Be Stored?
Valdoxan should be stored in a cool, dry place, away from direct sunlight. Always keep medications out of reach of children.
Can I Drink Alcohol While Taking Valdoxan?

It’s generally recommended to avoid alcohol while taking any antidepressant, including Valdoxan, as it can increase the risk of side effects and reduce the effectiveness of the medication.
Can I Stop Taking Valdoxan if I Feel Better?
It’s important not to stop taking Valdoxan without consulting your healthcare provider, even if you feel better. Abruptly stopping can lead to withdrawal symptoms and a potential relapse of depression symptoms.
Conclusion
As the first melatonergic antidepressant, Valdoxan is also the first new pharmacologic approach to depression since the SSRIs. Its novel mode of action has been shown to answer most of the unmet needs in depression therapy. By restoring biological rhythms, Valdoxan acts at the very core of depression, providing potent short- and long-term efficacy, exemplified in severely depressed patients, and outperforming the standard therapeutic options in this regard, namely SSRIs and SNRIs.
The regulation of sleep-wake rhythms enables Valdoxan to relieve the disturbed sleep experienced by depressed patients and to do so uniquely—not only without sedation or a hangover effect but with increased daytime alertness and preserved daily activities, all from the very first treatment week.
This novel mode of action also accounts for the favorable side-effect profile of Valdoxan. The striking absence of weight gain, sexual dysfunction, and discontinuation syndrome helps to secure unmatched levels of treatment adherence.47
Conventional treatments achieve remission in only a third of depressed patients. Even then the remission is often of poor quality due to residual symptoms. Its properties make Valdoxan unique in the antidepressant armamentarium.
It improves the quality of life during treatment and achieves better quality remission by acting directly on residual symptoms. As such, Valdoxan may well represent the antidepressant medication that patients and the psychiatric community have been waiting for.
References
1. Moeller HJ. Outcomes of major depressive disorder: the evolving concept of remission and its implications for treatment. World J Biol Psychiatry. 2008;9:102-114.
2. Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281: 1640-1645.
3. Griebel G, Simiand J, Steinberg R, et al. 4-(2-Chloro- 4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl- 1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2- propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR 125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333-345.
4. Souetre E, Salvati E, Wehr TA, et al. Twenty-four hour profiles of body temperature and plasma TSH in bipolar patients during depression and during remission and in normal control subjects. Am J Psychiatry. 1988;145:1133-1137.
5. Souetre E, Salvati E, Belugou JL, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263-278.
6. Wirz-Justice A. Biological rhythms in mood disorders. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press; 1995:999-1017.
7. Audinot V, Mailliet F, Lahaye-Brasseur C, et al. New selective ligands of human cloned melatonin MT1 and MT2 receptors. Naunyn-Shmiedeberg’s Arch Pharmacol. 2003;367:553-561.
8. Millan MJ, Gobert A, Lejeune F, et al. The novel melatonin agonist Valdoxan (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003;306:954-964.
9. Hanoun N, Mocaër E, Boyer PA, et al. Differential effects of the novel antidepressant Valdoxan (S 20098) versus fluoxetine on 5-HT1A receptors in the rat brain. Neuropharmacology. 2004;47:515-526.
10. Millan MJ, Brocco M, Gobert A, et al. Anxiolytic properties of Valdoxan, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology. 2005;177: 1-12.
11. Papp M, Gruca P, Boyer PA, et al. Effect of Valdoxan in the chronic mild stress model of depression in the rat. Neuropsychopharmacology. 2003;28:694-703.
12. Bertaina-Anglade V, Drieu la Rochelle C, Boyer PA, et al. Antidepressant-like effects of agomelatine (S 20098) in the learned helplessness model. Behav Pharmacol. 2006;17:703-713.
13. Barden N, Shink E, Labbe M, et al. Antidepressant action of Valdoxan (S 20098) in a transgenic mouse model. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:908-916.
14. Bourin M, Mocaer E, Porsolt R. Antidepressant-like activity of S 20098 (Valdoxan) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci. 2004;29: 126-133.
15. Van Reeth O, Weibel L, Olivares E, et al. Melatonin or a melatonin agonist corrects age-related changes in circadian response to environmental stimulus. Am J Physiol Regul Integr Comp Physiol. 2001;280: R1582-R1591.
16. Armstrong SM, McNulty OM, Guardiola-Lemaitre B, et al. Successful use of S20098 and melatonin in an animal model of delayed sleep-phase syndrome (DSPS). Pharmacol Biochem Behav.1993;46:45-49.
17. Redman JR, Guardiola-Lemaitre B, Brown M, et al. Dose-dependent effects of S-20098, a melatonin agonist, on the direction of re-entrainment of rat circadian activity rhythms. Psychopharmacology. 1995;118: 385-390.
18. Grassi-Zucconi G, Semprevivo M, Mocaër E, et al. Melatonin and its new agonist S 20098 restore synchronized sleep fragmented by experimental trypanosome infection in the rat. Brain Research Bulletin. 1996;39:63-68.
19. Corbach S, Schmelting B, Fuchs E, et al. Comparison of agomelatine and melatonin for effects in chronically stressed tree shrews, an animal model of depression. Eur Neuropsychopharmacol. 2007;7(suppl 4):S364.
20. Fuchs E, Corbach-Söhle S, Schmelting B, et al. Effects of agomelatine compared to melatonin in psychosocially stressed tree shrews. Int J Neuropsychopharmacol. 2008;11(suppl 1):121.
21. Leproult R, Van Onderbergen A, L’Hermitte-Balériaux M, et al. Phase shifts of 24-h rhythms of hormonal release and body temperature following early evening administration of the melatonin agonist agomelatine in healthy older men. Clin Endocrinol. 2005; 63:298-304.
22. Papp M, Litwa E, Gruca P, et al. The anxiolytic-like activity of Valdoxan and melatonin in three animal models of anxiety. Behav Pharmacol. 2006;17:9-18.
23. Banasr M, Soumier A, Hery M, et al. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006; 59:1087-1096.
24. Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275-317.
25. Moyer RW, Kennaway DJ. Immunohistochemical localization of serotonin receptors in the rat suprachiasmatic nucleus. Neurosci Lett. 1999;271:147-150.
26. Larson J, Jessen RE, Uz T, et al. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23-26.
27. Dugovic C, Leysen JE, Wauquier A. Melatonin modulates the sensitivity of 5-hydroxytryptamine-2 receptor-mediated sleep-wakefulness regulation in the rat. Neurosci Lett. 1989;104:320-325.
28. Kennaway DJ, Moyer RW. Serotonin 5-HT2C agonists mimic the effect of light pulses on circadian rhythms. Brain Res. 1998;806:257-270.
29. Holmes MC, French KL, Seckl JR. Dysregulation of diurnal rhythms of serotonin 5HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J Neurosci. 1997;17:4056-4065.
30. Masana MI, Benloucif S, Dubocovich ML. Circadian rhythm of MT1 melatonin receptor expression, in the suprachiasmatic nucleus of the C3H/HeN mouse. J Pineal Res. 2000;28:185-192.
31. Smith MI, Piper DC, Dixon MS, et al. Effect of SB- 243213, a selective 5-HT2C receptor antagonist, on the rat sleep profile: a comparison to paroxetine. Pharmacol Biochem Behav. 2002;71:599-605.
32. Lôo H, Hale A, D’Haenen H. Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT2C antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. Int Clin Psychopharmacol. 2002;17:239-247.
33. Olié JP, Kasper S. Efficacy of Valdoxan, an MT1/ MT2 receptor agonist with 5-HT2C antagonistic properties, in major depressive disorder. Int J Neuropsychopharmacol. 2007;10:661-673.
34. Kennedy SH, Emsley R. Placebo-controlled trial of Valdoxan in the treatment of major depressive disorder. Eur Neuropsychopharmacol. 2006;16:93-100.
35. Montgomery SA, Kasper S. Severe depression and antidepressants. Focus on a pooled analysis of placebo-controlled studies on agomelatine. Int Clin Psychopharmacol. 2007;22:283-291.
36. Goodwin GM, Rouillon F, Emsley R. Long-term efficacy of agomelatine, a novel antidepressant, in the prevention of relapse in out-patients with major depressive disorder. Eur Neuropsychopharmacol. 2007; 17(suppl 4):361.
37. Goodwin GM, Rouillon F, Emsley R. Long-term treatment with agomelatine: prevention of relapse in patients with major depressive disorder over 10 months. Eur Neuropsychopharmacol. 2008;18(suppl 4):S338.
38. Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine. J Clin Psychiatry. 2007;68:1723-1732.
39. Kasper S, Laigle L, Baylé F. Superior antidepressant efficacy of agomelatine versus sertraline: a randomized, double-blind study. Eur Neuropsychopharmacol. 2008;18(suppl 4):336.
40. Kennedy S, Lôo H, Olié JP. The antidepressant efficacy of agomelatine is independent of gender, age, body mass index, and use of concomitant anxiolytics: combined analysis of three placebo-controlled studies. Int J Neuropsychopharmacol. 2008;11(suppl 1):193.
41. Guilleminault C, Quera-Salva MA. Impact of the melatonergic antidepressant Valdoxan on sleep-wake rhythms of depressed patients. Eur Neuropsycho- pharmacol. 2006;16(suppl 4):317.
42. Quera Salva MA, Vanier B, Laredo J, et al. Major depressive disorder, sleep EEG and agomelatine: an open-label study. Int J Neuropsychopharmacol. 2007; 10:691-696.
43. Kasper S. Effect of agomelatine on rest-activity cycle in patients with major depressive disorder compared to sertraline. Int J Neuropsychopharmacol. 2008;11(suppl 1):193.
44. Kennedy SH, Rizvi S, Fulton K, et al. A double-blind comparison of sexual functioning, antidepressant efficacy, and tolerability between agomelatine and venlafaxine XR. J Clin Psychopharmacol. 2008; 28:329-333.
45. Montejo AL, Prieto N, Terleira A, et al. Better sexual acceptability of agomelatine (25 and 50 mg) compared to paroxetine (20 mg) in healthy male volunteers. An 8-week, placebo-controlled study using the PRSEXDQ-SALSEX scale. J Psychopharmacol. Sept 18, 2008. Epub ahead of print.
46. Montgomery SA, Kennedy SH, Burrows GD, et al. Absence of discontinuation symptoms with Valdoxan and occurrence of discontinuation symptoms with paroxetine: a randomized, double-blind, placebo-controlled discontinuation study. Int Clin Psychopharmacol. 2004;19:271-280.
47. Rouillon F. Efficacy/tolerance profile of Valdoxan and practical use in depressed patients. Eur Neuropsychopharmacol. 2005;15:S660-S661.
AMÉLIORER LA QUALITÉ DE LA RÉMISSION DANS LA DÉPRESSION:
VALDOXAN, LE PREMIER ANTIDÉPRESSEUR MÉLATONINERGIQUE
Le fait que les antidépresseurs classiques confèrent rarement une rémission de bonne qualité suggère que d’autres modèles que le système monoaminergique pourraient ouvrir la voie à un traitement efficace. La régulation des rythmes circadiens en est l’illustration, fondée sur la perturbation de ces rythmes chez les patients déprimés et leur l’importance dans l’évolution des l’épisodes dépressifs.
Valdoxan (agomélatine) est le premier antidépresseur mélatoninergique. Son profil d’action au niveau des récepteurs lui confère une efficacité unique dans la régulation des troubles des rythmes circadiens. Valdoxan, en effet, est le premier antidépresseur agoniste des récepteurs mélatoninergiques MT1 et MT2 et antagoniste des récepteurs sérotoninergiques 5HT2C.
Son efficacité antidépressive a été démontrée à court et long termes contre placebo, quelle que soit la sévérité de la maladie, et s’est avérée supérieure à celle de la venlafaxine et la sertraline aux différentes échelles d’évaluation, particulièrement celles utilisées en pratique clinique. Cette efficacité s’associe à des bénéfices cliniques singuliers tels que la régulation non sédative du cycle veille-sommeil et l’amélioration du fonctionnement diurne, dès la première semaine de traitement.
Valdoxan n’altère pas les fonctions sexuelles ni la libido, il n’induit pas de syndrome de manque à l’arrêt du traitement ou de prise de poids, et il est bien toléré, ce qui en fait un traitement réellement innovant de la dépression, permettant un soulagement précoce des symptômes ainsi qu’une rémission complète et prolongée.




