The first step towards applying evidence-based healthcare is to adopt international guidelines for disease characterization and gauging the results of treatments. This review article aims to shed light on the latest guidelines, particularly those concerning the medicinal management of chronic venous conditions.
We present an overview of the universally accepted disease definitions, standardized terminology, patient symptoms, the severity of venous conditions, and the proven measures of success post-treatment.
The article also delves into the frequency, impact, and physiological roots of chronic venous disorders, as well as the categorization of the most commonly prescribed venoactive medications. It’s clear that venous hypertension is a common thread in all clinical symptoms of the disease.
Inflammation plays a pivotal role in the remodeling of the vein wall, valve malfunction, and ensuing venous hypertension, which then affects the microcirculation. This leads to changes in the capillaries, resulting in swelling, skin alterations, and ultimately, venous ulcers.
Venous symptoms could be a consequence of the interaction between pro-inflammatory agents and nerve fibers situated around the capillaries. Hence, inflammation in the veins is a prime target for drug intervention, especially for venoactive medications.
We then discuss recent guidelines that have assessed the advantages of venoactive drugs, as seen in reviews, book chapters, and international guidelines on managing chronic venous disorders. Recent research, reviews, and meta-analyses strongly advocate for the use of micronized purified flavonoid fraction (Daflon 500 mg).
There’s still work to be done to update the guidelines on managing chronic venous disorders using venoactive drugs. There’s a particular emphasis on the need for more extensive and conclusive clinical trials to enhance the current recommendations.
Medicographia. 2015;37:71-79 (see French abstract on page 79)
This article addresses some of the newer guidelines on vasoactive drugs (VADs) in general, and Daflon 500 mg in particular, in the management of chronic venous disorders, to help clinicians better manage patients with venous disorders of the lower extremity.
Intentionally, only the primary disease will be tackled in this review, putting postthrombotic venous disease aside.
A common language is needed before building guidelines in chronic venous disorders
It should first be stressed that no consensus on guidelines is possible without the use of a common language. A leap forward was recently made thanks to: (i) a common terminology on venous anatomy,1 the clinical, etiological, anatomical, pathophysiological (CEAP) classification proposed by the ad hoc committee of the American Venous Forum in 1994 and revised in 2004,2 which was subsequently adopted worldwide as a basis for improved patient description; and (ii) a consensus on terminology related to chronic venous disorders to avoid misunderstanding and lack of precision in publications.
The last consensus document (VEIN Term) provides the definition of 33 widely used clinical venous terms and was published in The Journal of Vascular Surgery, 2009, under the aegis of the main American and European scientific societies (American Venous Forum, American College of Phlebology, European Venous Forum, Union Internationale de Phlébologie [UIP; International Union of Phlebology], International Union of Angiology, and Society for Vascular Surgery).3
The CEAP classification includes a clinical assessment (C), an etiological assessment of the patient’s disease (E), an anatomical assessment of the location of the pathology (A), and the pathophysiological basis for the underlying disease (P). It provides a broad-based, objective, anatomic, and physiologic basis for the classification of venous disease. This is why CEAP has improved standardization, communication, decision-making, and reporting of venous disease.
What does the term “chronic venous disorders” cover?
The term “chronic venous disorders” covers a full spectrum of venous conditions ranging from patients with symptoms only (C0s of the CEAP classification) and telangiectasias to the ultimate complications, venous ulcers. Symptoms are commonly associated with signs of chronic venous disorders.
Venous symptoms are defined as tingling, aching, burning, pain, muscle cramps, swelling, sensations of throbbing or heaviness, itching skin, restless legs, leg tiredness and/or fatigue, all of which may be exacerbated during the course of the day or by heat, but relieved with leg rest, elevation, or both.3
Venous signs are visible manifestations of chronic venous disorders, which include dilated veins (telangiectasias, reticular veins, varicose veins), leg edema, skin changes, and ulcers, as described in the CEAP classification.2

Chronic venous disorders include those patients with symptoms only, but presenting no signs at clinical examination or ultrasound investigation (the so-called C0s patients), and those with venous signs as described in the CEAP classification. The latter may be either symptomatic or asymptomatic.
The burden of chronic venous disorders
Chronic venous disorders are common conditions in Western countries that have a significant impact on affected individuals and the health care system. In the French survey by Carpentier et al,4 the percentage of symptomatic patients with chronic venous disease (CVD) varied between 25% and 84%, depending on the severity of the disease (the magnitude of symptoms increased with severity).
According to population-based epidemiological studies in various countries, telangiectasias and reticular veins are present in approximately 80% of men and 85% of women, and varicose veins in 25% to 32% of women and 7% to 40% of men. The prevalence of open plus healed venous ulceration is estimated at approximately 1% of the population.5
In the recent Vein Consult Program initiated with the UIP, which surveyed 91 545 subjects of 20 countries worldwide, the prevalence of chronic venous disorders was 83.6%: 63.9% of the subjects ranging from C1 to C6, and 19.7% being C0s subjects. C0s patients were more frequently men, whatever the age or geographical zone. C1 to C3 appeared to be more frequent among women, whatever the country, but the rate of severe stages (C4 to C6) did not differ between men and women.6
The high prevalence of varicose veins and the chronicity of leg ulcers mean that CVD has a considerable impact on health care resources. It has been estimated that venous ulcers cause the loss of approximately 2 million working days and incur treatment costs of approximately $3 billion per year in the United States. In European countries, medical care costs associated with the disease have been estimated to account for 1% to 3% of total annual health care budgets.7
CVD is associated with a reduced quality of life, particularly in relation to pain, physical function, and mobility. It is also associated with depression and social isolation. The impairment associated with venous leg ulcers, the most severe manifestation of CVD, has been likened to the impairment associated with stage II to III of heart failure.8
The main categories of venoactive drugs

VADs, also known as phlebotonic agents, are often used to treat chronic venous disorders. Main classes of VADs include coumarin, flavonoids, saponins, and synthetic products (calcium dobesilate, benzaron, and naftazon). On the basis of randomized controlled trials (RCTs) and meta-analyses, previous guidelines concluded that VADs should be used as an adjunct treatment for symptomatic C0 to C6 chronic venous disorders except in specific situations, such as hot climates, where they may be used in place of compression.9 Flavonoids are VADs of particular interest in the treatment of chronic venous disorders. Flavonoids were initially described as “vitamin P” (for permeability) because their deficiency causes capillary fragility and increases vessel wall permeability. Flavonoids are naturally occurring polyphenolic compounds widely found in nature, especially as plant pigments.
Main classes include flavones, flavonols, flavanes, flavanones, anthocyanadins, isoflavonoids, and neoflavonoids. Flavonoid products used for the treatment of chronic venous disorders include micronized purified flavonoid fraction (MPFF), oxerutin, and Ο– (β-hydroxyethyl) rutosides (Table I).5,10
MPFF contains purified flavonoids, mostly hesperidin and diosmin, from Rutaceae aurantiae (orange) micronized into 2 mm particles to help improve intestinal absorption. Oxerutin and Ο– (β-hydroxyethyl) rutosides are derived by hydroxyethylation of rutin, a naturally occurring glycoside of quercetin and the disaccharide rutinose. Red vine leaf extract is a preparation made from the leaves of wine grape (Vitis vinifera) containing the flavonols quercetin glucoside, quercetin glucuronides, and kaempferol glucoside. Pycnogenol is an extract of maritime pine bark containing proanthocyanidins, which are polymers of flavonoids.9
Pathophysiological mechanisms and pharmacological treatment of chronic venous disorders
Valve and vein wall changes
Results from studies that demonstrate treatment efficacy lead to guideline recommendations. Ambulatory venous hypertension is the hemodynamic disease that is related to all symptoms and signs of chronic venous disorders, the underlying components of venous hypertension mainly being failure of the calf muscle pump, venous valvular incompetence, and luminal obstruction.11,12
Venous hypertension is the underlying cause of chronic venous disorders and lies in the complex cellular and molecular processes set in motion by abnormal venous hemodynamics. When venous pressures in the leg reach higher-than-normal levels and remain elevated for prolonged periods, a progressive increase in skin damage occurs.
Nicolaides reported that nearly all patients with exercising venous pressures of >90 mm Hg experienced venous ulceration.13 Primary chronic venous disorders are the result of increased and unabated venous hypertension caused mostly by reflux through incompetent valves. To be efficient, any treatment should prevent or decrease superficial valve incompetency in order to counteract venous hypertension. It is only recently that research interest has focused on the action of VADs on chronic inflammatory processes that can affect large and small venous vessels and valves of the superficial venous system.
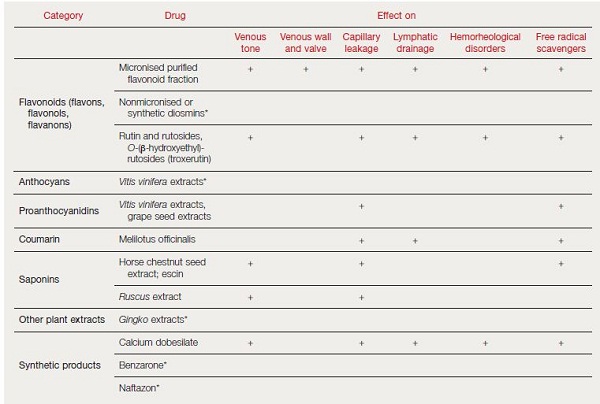
- Data from references 5 and 10
Evidence has accumulated over the past years showing that inflammation could be key in wall remodeling, valve failure, and subsequent venous hypertension.11,14 Various types of inflammatory mediators and growth factors are released (Figure 1), including vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), transforming growth factor β1 (TGF-β1), fibroblast growth factor β1 (FGF-β1), and vascular endothelial growth factor (VEGF).
The inflammatory cascades in the vein wall and venous valves can cause progressive valvular incompetence and eventual valvular destruction.12 Once initiated, venous valve damage will be self-reinforcing, exacerbating venous hypertension and disturbance of venous flow, and causing further inflammation. As a result, reflux appears and may occur in the superficial or deep venous system or in both.
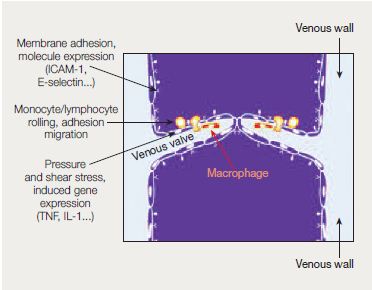
Inflammatory gene expression in the endothelium may be induced by a shift in venous hydrostatic pressure and fluid shear stress. This supports leukocyte rolling, adhesion, and migration, together with free radical formation, apoptosis, and tissue necrosis. In the process, macrophages become the instruments of tissue damage.
- Abbreviations: ICAM-1, intercellular adhesion molecule 1; IL, interleukin; TNF, tumor necrosis factor.
- After reference 12: Perrin M, Ramelet AA. Eur J Vasc Endovasc Surg. 2011; 41(1):117-125. © 2010 European Society for Vascular Surgery. Published by Elsevier Ltd. All rights reserved.
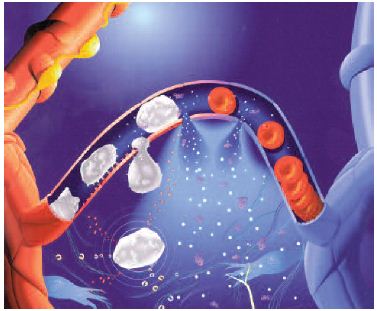
Venous hypertension is transmitted to the microcirculation and prompts leukocyte adhesion to capillary endothelium. This initiates an inflammatory reaction which dramatically increases capillary permeability. When transcapillary filtration exceeds lymphatic flow, interstitial edema occurs.
Flavonoids are known to have potent antioxidant properties that have been investigated in several therapeutic areas other than CVD, including cancer, arthritis, and cardiovascular disease.15 More specifically, the VADs MPFF and rutosides have shown powerful free-radical scavenging properties in various assay systems,15 and VADs from other groups have also shown similar properties, including escins, proanthocyanidines from grape seeds and French maritime pine bark, and calcium dobesilate.5
In addition to actions that reduce oxidative stress, several VADs also act at various points in inflammatory cascades. As examples, grape seed proanthocyanidin reduced expression of cell adhesion molecules by activated cultured vein endothelial cells, and MPFF decreased expression of adhesion molecules by neutrophils and monocytes in patients with chronic venous disorders.5
Capillary alteration
Venous hypertension increases hydrostatic pressure in capillaries resulting in transcapillary filtration that exceeds lymphatic flow. This contributes to the formation of interstitial edema. Venous hypertension alters blood flow in capillaries, prompting leucocyte adhesion to capillary endothelium and initiating an inflammatory reaction.16 The capillary gaps would become very large, greatly raising capillary permeability to fluid, macromolecules, and extravasated red blood cells, resulting in their flow into the interstitial space and in edema formation (Figure 2).12
Fragmentation and destruction of lymphatic vessels may further impair drainage from the extremity, whereas dysfunction of local nerve fibers may alter regulatory mechanisms.11 Given their antioxidant and anti-inflammatory effects, it is not surprising that many of the major VADs have been shown to reduce capillary hyperpermeability, including MPFF, rutosides, escin, Ruscus extract, grape seed extract, and calcium dobesilate.5
Skin changes and venous ulceration
Several mechanisms for the development of venous ulceration have been postulated, of which the theory of “leucocyte trapping” is the most likely,17 although challenged today. It is hypothesised that the primary injury to the skin (which is the final target of chronic venous hypertension) is extravasation of macromolecules, such as fibrinogen and α-macroglobulin, and red blood cells into the dermal interstitium.
Red blood cell degradation products and extravasated interstitial protein are potent chemoattractants and presumably generate the initial inflammatory signal, which results in leukocyte recruitment and migration into the dermis. Pathologic events occur during leucocyte migration into the dermis and the end product of these is dermal fibrosis.
A cascade of inflammatory events results in cutaneous changes, which include skin hyperpigmentation caused by hemosiderin deposition and eczematous dermatitis. Fibrosis may develop in the dermis and subcutaneous tissue (lipodermatosclerosis).
Interest in the mechanisms underlying skin changes has received new impetus with the increasing recognition of the importance of venous valves in small veins and venules. It is now appreciated that small superficial veins of the human lower limb contain abundant, typical bicuspid venous valves, with the majority occurring in vessels less than 100 μm in diameter and present in vessels as small as 18 μm.
A recent study has shown that incompetence can occur in human small superficial venous valves independently of reflux within the great saphenous vein and major tributaries. Importantly, degenerative changes and incompetence in these microvenous valves can allow reflux into the microvenous networks in the skin, which may be critical in the development of severe skin changes in chronic venous disorders.18
- Data from references 12, 15, 17, and 20.
The ability of VADs to reduce inflammation and oxidative stress could protect small venous valves and prevent reflux, and also act at the level of preventing the adverse remodeling of skin tissue that ultimately may lead to the development of active ulcers in chronic venous disorders.
Symptoms and the role of C nociceptors
Typical leg symptoms of chronic venous disorders are common in those with even the least severe forms (CEAP C0s and C1). In a recent report from the Vein Consult Program, a large cohort of over 90 000 consecutive outpatients from 20 countries, who were consulting their general practitioner for any reason, were screened for chronic venous disorders. Of these, 19.7% had typical chronic venous disorder leg symptoms without signs and were assigned to CEAP class C0s, and a further 21.7% were assigned to class C1.6 The exact mechanisms by which chronic venous disorders, particularly the earliest stages, give rise to pain and other typical venous symptoms are not yet understood, but recent studies suggest inflammation plays a key role.19,20 Sympathetic C fibers are found in the venous intima and media, and wrapped around cutaneous venules, and act as nociceptors that can respond to inflammatory mediators.
Inflammatory processes seem to be involved in all stages and severities of chronic venous disorders, even before obvious tissue damage has occurred, and could be responsible for many of the symptoms experienced. Thus, the anti-inflammatory properties of VADs have the potential to improve symptoms in patients at all stages of the disease, including those in CEAP class C0s.
Lymphatic drainage
Lymphatic function is known to be compromised in patients with especially the more advanced stages of chronic venous disorders, and has been shown to improve in patients with varicose veins after reduction of venous reflux by saphenous vein ablation.21 Several VADs, including alpha-benzopyrones (coumarin) either alone or combined with rutin,22,23 MPFF,24 and calcium dobesilate25 have all been shown to improve lymphatic drainage in animal models.
Hemorheological disorders
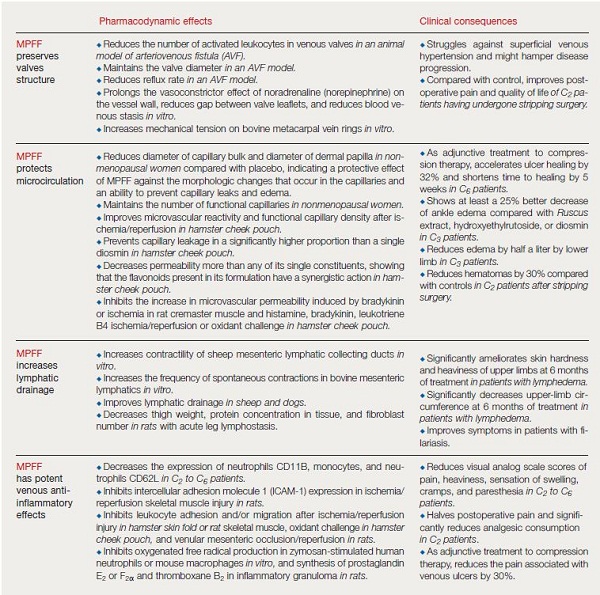
Hemorheological changes, including increased blood viscosity and erythrocyte aggregation, are common in chronic venous disorders. Several VADs have been shown to reduce blood viscosity and/or erythrocyte aggregation, including MPFF,26 troxerutin27 and calcium dobesilate.28 The pharmacological effects of VADs are summarized in Table I.5,10 The mode of action more specifically related to MPFF is described in Table II (page 75).12,13,17,20
- Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
- Data from reference 29.
A new grading system for recommendations in guidelines
The method of determining the strength and quality of the recommendations in American guidelines deserves mention. Recommendations are generally accompanied by a number, which refers to the strength of the recommendation, and a letter, which refers to the quality of the evidence supporting the recommendation. Recent guidelines for venous disease have used two levels for the strength of their recommendations depending mainly on the benefit/risk ratio: grade 1 for strong and grade 2 for weak. They further indicate that statements accompanied by a grade 1 level are “recommendations” and statements accompanied by a grade 2 level are “suggestions” (Table III).29
The quality of evidence upon which the strength of the recommendation is based ranges from “A” for high quality, which is consistent evidence from randomized trials, to “B” for moderate quality, which is evidence from nonrandomized trials or inconsistent evidence from randomized trials. Level “C” is low quality, which is suggestive evidence from nonrandomized trials, observational reports, or expert opinion. Writing committees are increasingly aware of the cost of care and patient values and preferences, as are physicians. These are also considered in the strength of recommendation.
The recent guidelines on the management of chronic venous disorders
Recent reviews and guidelines on chronic venous disorders have used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system (Figure 3):
- The article by Perrin et al published in The European Journal of Vascular and Endovascular Surgery, 2011, which reviews the evidence for pharmacological therapies of primary chronic venous disorders together with the rationale for such treatment and the questions that remain unanswered.12
- The latest (second) edition of The Vein Book edited by Bergan and Bunke, which covers the entire spectrum of venous conditions from clarification of the pathophysiology of chronic venous disorders, molecular mechanisms in the cause of varicose veins, new treatment options for varicose veins and spider veins, startling new treatment for venous thromboembolic disease, and effective treatment for leg ulcers.30
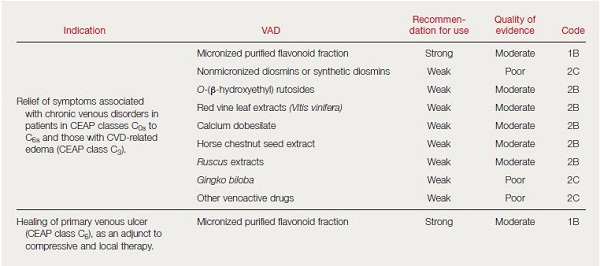
- The updated recommendations on the management of chronic venous disorders, results of a consensus conference inititiated by the European Venous Forum (EVF) and held in 2012 in Cyprus with renown experts. The consensus document was published under the auspices of the EVF, the International Union of Angiology (IUA), the UIP; and the Cardiovascular Disease Educational and Research Trust (CDIRT).10 In summary and based on the quality of evidence, the authors found it possible to propose a strong recommendation, based on evidence of moderate quality (1B), for the use of MPFF in symptoms and edema. Rutosides, horse chestnut seed extract, and Ruscus extracts have also proven effective against CVD-related symptoms and lower limb edema, although the volume and quality of evidence is less than for the previous drug.

- Data from references 10, 12, and 30.
Updating guidelines on chronic venous disorders
An update of the Guidelines for Testing Drugs for Chronic Venous Insufficiency is needed to allow the pharmaceutical industry investing the necessary resources to perform large and definitive clinical trials that could improve the recommendations, which are useful for clinicians and organizations involved in decision making in this important field of chronic venous disorders. Such guidelines could:
- Reiterate the basic principles that should prevail when reporting from (and setting up) any RCT, using the Consolidated Standards of Reporting Trials (CONSORT) statement,31 as for meta-analyses with the QUORUM checklist.32
- Abbreviations: CEAP, clinical, etiological, anatomical, and pathophysiological classification; GRADE, Grading of Recommendations Assessment, Development and Evaluation.
- Comprehensively describe patients at selection in a study, using the advanced CEAP classification,2 which implies that all classes of the CEAP must be completed, and that duplex scan investigation, with or without plethysmography (level 2 investigation) is mandatory.
- Include larger sample sizes (>200 patients in each group), having in mind the high incidence of the placebo effect.12 For instance, the most recent studies on VADs included only between 30 and 125 patients in each group.9
- Promote the use of validated tools to assess symptoms, edema, and venous leg ulcers, and have a consensus on end points.33
- Encourage the adoption of a simple and universally understood system of grading.29
- Perform long-term studies in order to examine the prevention of chronic venous disorder progression and assess the cost-effectiveness of VADs.9
Frequently Asked Questions
Why is there a need for more research on venoactive drugs like Daflon 500 mg?
While venoactive drugs like Daflon 500 mg have shown promising results, there is a need for more extensive and conclusive clinical trials. This will help enhance current recommendations and ensure the most effective treatment strategies for chronic venous disorders.
What are the potential side effects of Daflon 500 mg?
As with any medication, Daflon 500 mg may have potential side effects. It’s important to consult with a healthcare provider to understand the potential risks and benefits before starting any new medication.
How prevalent are chronic venous disorders?
Chronic venous disorders are common conditions in Western countries, significantly impacting affected individuals and the healthcare system. They can range from minor symptoms to severe complications like venous ulcers.
Are there any dietary restrictions or lifestyle changes recommended when taking Daflon 500 mg?
While Daflon 500 mg is generally well-tolerated, it’s always a good idea to discuss any dietary restrictions or recommended lifestyle changes with your healthcare provider.
What are the long-term effects of using Daflon 500 mg for chronic venous disorders?
The long-term effects of Daflon 500 mg are still being studied. However, it has shown promising results in managing symptoms and improving the quality of life for patients with chronic venous disorders.
Final Words
Daflon 500 mg plays a significant role in managing chronic venous disorders. Its effectiveness in reducing symptoms and improving patient quality of life makes it a valuable tool in the treatment arsenal. However, as with any medication, it’s essential to consult with a healthcare provider to understand its potential benefits and risks. As research continues, we can look forward to more refined treatment strategies and improved patient outcomes in the future.
References:
1. Caggiati A, Bergan JJ, Gloviczki P, et al. Nomenclature of the veins of the lower limbs: an international interdisciplinary consensus statement. J Vasc Surg. 2002;36:416-422.
2. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40: 1248-1252.
3. Eklof B, Perrin M, Delis K, Rutherford R. Updated terminology of chronic venous disorders: the Vein Term Transatlantic Interdisciplinary Consensus Document. J Vasc Surg. 2009;49:498-501.
4. Carpentier PH, Maricq HR, Biro C, Poncot-Makinen CO, Franco A. Prevalence, risk factors and clinical patterns of chronic venous disorders of lower limbs. A population-based study in France. J Vasc Surg. 2004;40:650-659.
5. Nicolaides A, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Int Angiol. 2008;27:1-59.
6. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F; VCP coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31:105-115.
7. Beebe-Dimmer JL, Pfeifer J, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175- 184.
8. Andreozzi GM, Cordova RM, Scomparin A, Martini R, D’Eri A, Andreozzi F. Quality of life in chronic venous insufficiency. An Italian pilot study of the Triveneto Region. Int Angiol. 2005;24:272-277.
9. Rabe E, Guex JJ, Morrison N, et al. Treatment of chronic venous disease with flavonoids: recommendations for treatment and further studies. Phlebology. 2013;28(6):308-319.
10. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs . Guidelines according to scientific evidence. Int Angiol. 2014:33 (2):126-139.
11. Bergan JJ, Schmid-Schönbein G, Coleridge-Smith P, Nicolaides A, Boisseau M, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-498.
12. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41(1):117-125.
13. Nicolaides AN, Hussien MK, Szendro G, et al. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414-419.
14. Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85-98.
15. Lyseng-Williamson A, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
16. Boisseau MR. Leukocyte involvement in the signs and symptoms of chronic venous disease. Perspectives for therapy. Clin Hemorrheol Microcirc. 2007;37: 277-290.
17. Saharay M, Shields DA, Porter JB, Scurr JH, Coleridge-Smith PD. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;26:265-273.
18. Vincent JR, Jones GT, Hill GB, van Rij AM. Failure of microvenous valves in small superficial veins is a key to the skin changes of venous insufficiency. J Vasc Surg. 2011;54(6 suppl):62S-69S.
19. Danziger N. Pathophysiology of pain in venous disease [in French]. J Mal Vasc. 2007;32:1-7.
20. Vital A, Carles D, Serise JM, Boisseau MR. Evidence for unmyelinated C fibres and inflammatory cells in human varicose saphenous vein. Int J Angiol. 2010; 19:e73-e77.
21. Suzuki M, Unno N, Yamamoto N, et al. Impaired lymphatic function recovered after great saphenous vein stripping in patients with varicose vein: venody-namic and lymphodynamic results. J Vasc Surg. 2009;50:1085-1091.
22. Casley Smith JR. Modern treatment of lymphedema. II. The benzopyrones. Australas J Dermatol. 1992;33:69-74.
23. Casley Smith JR, Morgan RG, Piller NB. Treatment of lymphedema of the arms and legs with 5,6 benzo [alpha] pyrone. N Engl J Med. 1993;329:1158-1163.
24. Labrid C. A lymphatic function of Daflon 500 mg. Int Angiol. 1995;14(3 suppl 1):36-38.
25. Piller NB. The lymphagogue action of calcium dobesilate on the flow of lymph from the thoracic duct of anesthetized and mobile guinea pigs. Lymphology. 1988;21:124-127.
26. Le Dévéhat C, Khodabandehlou T, Vimeux M, Kempf C. Evaluation of haemorheological and microcirculatory disturbances in chronic venous insufficiency: activity of Daflon 500 mg. Int J Microcirc Clin Exp. 1997;17 suppl 1:27-33.
27. Boisseau MR, Taccoen A,Garreau C,Vergnes C,Roudaut MF,Garreau-Gomez B. Fibrinolysis and hemorheology in chronic venous insufficiency: a double blind study of troxerutin efficiency. J Cardiovasc Surg (Torino). 1995;36:369-374.
28. Benarroch IS, Brodsky M, Rubinstein A, Viggiano C, Salama EA. Treatment of blood hyperviscosity with calcium dobesilate in patients with diabetic retinopathy. Ophthalmic Res. 1985;17:131-138.
29. Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American college of chest physicians task force. Chest. 2006;129;174-181.
30. Perrin M, Ramelet AA. Efficacy of venoactive drugs in primary chronic venous disease. Survey of evidence, synthesis and recommendations. In: Bergan JJ, Bunke N, eds. The Vein Book. 2nd ed. New York, NY: Oxford University Press; 2014:514-527.
31. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomized trials. BMJ. 2010;340:869.
32. Clarke M. The QUORUM statement. Lancet. 2000;355:756-757.
33. Vasquez MA, Munschauer CE. Venous Clinical Severity Score and quality-of-life assessment tools: application to vein practice. Phlebology. 2008;23:259-275.




